Wetting superhydrophobic surfaces
Superhydrophobic surfaces, which are useful in various applications, can be prepared easily by decorating a hydrophobic surface (e.g. polymer material) with physical roughness (e.g. pillars). A water droplet in contact with a superhydrophobic surface of this kind rests on the top of the pillars, like a Fakir on a bed of nails (this situation is often termed "Cassie-Baxter state"). Since the droplet just touches the apex of the pillars, it is essentially in contact with the air in between the pillars, which makes the droplet completely spherical and enables it to move around freely. As an additional advantage, repelled water droplets can take away small dirt particles while rolling off the surface, i.e. the surface is self-cleaning. This effect is called the ”Lotus effect”, after the most well-known example present in Nature: the Lotus leave. Note that also animals utilize superhydrophobicity, e.g. blue swallows to fly in thick mist, water striders to walk on water, and butterflies to keep their fragile wings clean and dry.
Surprisingly, however, it may be energetically more favorable for the droplet to wet the structure completely (termed "Wenzel state"). The reason that this does not occur spontaneously, is due to an energy barrier associated with wetting the surface structure in the vertical direction.
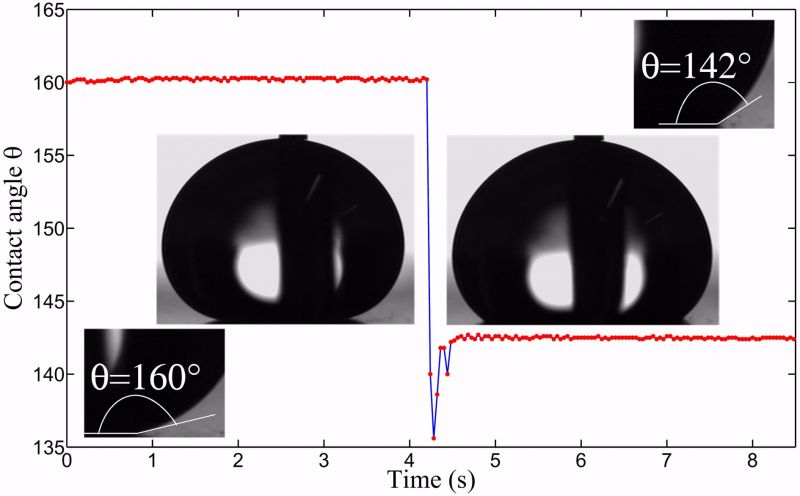
Figure 1: Transition from the (meta-)stable CB to the W state. A drop softly deposited on a micro-patterned surface can stay suspended with air pockets trapped in the grooves underneath the liquid (left). At some point the CB state spontaneously breaks down. The drop then homogeneously wets the substrate, resulting in a lower contact angle (right).
In this project we have studied the wetting characteristics of a water droplet invading a superhydrophobic substrate. The initial energy barrier is overcome, either by a local defect in the micro-structure (spontaneous), or by sideways pulling the droplet into the structure (forced). Subsequently, an energy argument dictates whether or not and how the droplet will further wet the micro-structure. We find that the velocity of the wetting front diverges as the dimensions of the structure approach a critical value, with square-shaped wetted areas as a direct result. The resulting wetting phenomenon is doped "zipping wetting".
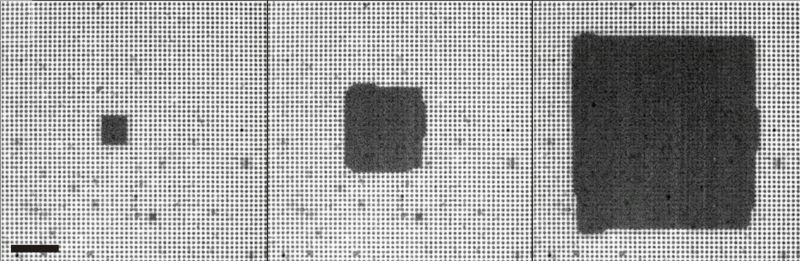
Figure 2: The microstructure underneath the liquid fills in a few hundreds of milliseconds through a square-like pattern involving a zipping wetting mechanism. Scalebar = 100 µm; t=36, 72 and 118 ms (from left to right, respectively).
Info: Detlef Lohse
Researchers: Bram Borkent, Mauro Sbragaglia, Christophe Pirat, Detlef Lohse.
Collaborators: Alisia Peters, Rob Lammertink, Matthias Wessling (Membrane Technology Group, U Twente)
Embedding: STW Nanoned Programme and Spearhead programme on Micro- and Nanofluidics
Sponsors: STW
